Lenacapavir's Promise Depends on Strong Health Systems
.png)
At this year's UN General Assembly, the global health community witnessed something extraordinary: a convergence of public health agencies, pharmaceutical innovators, and financing partners coming together around lenacapavir. This twice-yearly injectable PrEP for HIV was developed by Gilead Sciences.
The numbers are stunning: the current US price for lenacapavir is $28,000 annually. Through partnerships announced at UNGA – PEPFAR and the Global Fund working directly with Gilead, the Gates Foundation partnering with Hetero Labs, and Unitaid, CHAI, and Wits RHI partnering with Dr. Reddy's – the cost for low- and middle-income countries drops to $40 annually, with rollout starting in 2027. Some projections suggest costs could fall to one-thousandth of the US price.
What is lenacapavir – and what's so special?
What makes lenacapavir revolutionary is that a single injection offers six months of protection. Such a duration is transformative for adherence and a potential tool for overcoming PrEP stigma in underserved communities.
%20(1).jpg)
It's worth celebrating. But the truth is, affordability is only the first hurdle. The health systems required to deliver lenacapavir don't yet exist in many countries with the highest HIV burden. Without immediate, sustained investment in these systems, even the most affordable miracle drug will sit unused.
We've seen this story before. When antiretroviral therapy became affordable in the early 2000s, it took years to build delivery systems. In 2003, only about 400,000 people in LMICs were on ART; by 2009, that number grew to more than 5 million. That's still a fraction of the tens of millions requiring it.
The delay wasn't just about money but about systems. Innovation without infrastructure means delayed access, which means preventable infections, which means lives lost.
Invisible infrastructure of access
Getting a drug from "affordable" to "administered" requires interconnected systems that rarely make headlines:
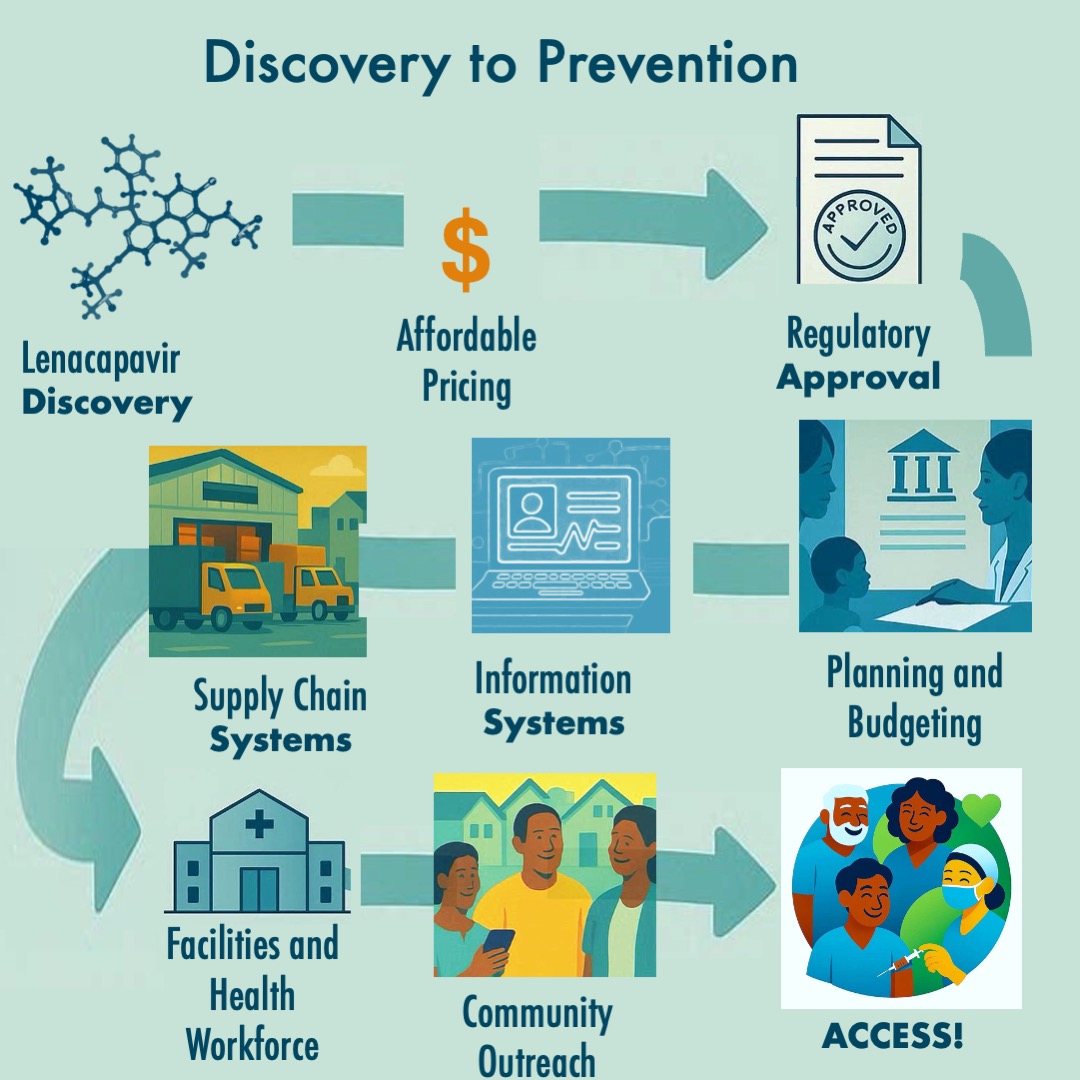
- Regulatory & Clinical Systems must review and approve lenacapavir (improving thanks to initiatives like the East African Community Medicines Regulatory Harmonization, which reduced review times from 553 days to 259), establish clinical protocols, ensure laboratory capacity for HIV testing, and train health workers to administer injections. South Africa’s successful efforts to fast track lenacapavir for approval should be celebrated.
- Supply Chain & Pharmaceutical Systems must forecast demand years ahead, ensure cold chain integrity, track inventory in real-time, coordinate last-mile delivery to vulnerable populations, and implement quality assurance to detect counterfeits.
- Data & Information Systems must create patient registries to track who received doses and when they're due for the next one – critical for a twice-yearly injection. These need interoperability between HIV programs, sexual health services, and primary care, plus pharmacovigilance to monitor adverse events.
- Community & Demand Systems need resourced community health workers for outreach and education, peer navigator programs for key populations facing stigma, culturally appropriate health literacy campaigns, and feedback mechanisms ensuring accountability.
- Financing & Governance Systems must integrate lenacapavir into insurance benefits, establish sustainable domestic financing as donor funding evolves, and create policy frameworks ensuring marginalized populations aren't left behind.
- Integration & Coordination with existing PrEP programs, maternal and child health platforms, HIV treatment programs, TB/hepatitis/STI prevention, and broader primary care services – each requiring coordination mechanisms and cross-trained staff.
And here's what makes lenacapavir particularly complex: it's not one-time. It requires sustained biannual re-engagement with health systems over years. That means robust patient tracking, reliable reminder systems, consistent supply chain performance, and maintained trust between health systems and communities.
A breakdown anywhere in this chain, from regulatory approval to last-mile delivery, means the drug doesn't reach the person who needs it.
The efficiency imperative
Strong systems ensure efficiency, making sure the right medicines are reaching the right populations at the right time. Weak health systems consistently fail the same populations: adolescent girls and young women, sex workers, men who have sex with men, transgender individuals, people who use drugs, migrants, and those in rural or conflict-affected areas.
These are precisely the populations who will benefit most from long-acting prevention. And targeting them efficiently improves prevention efforts. Without intentional systems strengthening that centers efficiency and is informed by lessons from stigma reduction, culturally competent service delivery, and deliberate outreach. Lenacapavir will follow the same unequal access patterns we've seen with other innovations.
What next for lenacapavir
The UNGA agreements represent visionary leadership. Here's what must happen in the next 12-24 months:
- Regulatory Fast-Tracking: Countries should prioritize improving regulatory efficiency, including it in negotiations for bilateral and multilateral support. PEPFAR and the Global Fund should immediately allocate funding for regulatory capacity building, building on the October 2025 WHO Prequalification for lenacapavir, advancing the WHO Stringent Regulatory Authority Collaborative Registration Procedure, supporting joint reviews through regional bodies, and establishing mutual recognition agreements.
- Supply Chain Infrastructure: Governments, bilateral donors and the Global Fund should commit HIV prevention budgets to digital inventory management providing real-time visibility, adequate cold chain infrastructure, last-mile delivery reaching key populations, and quality assurance preventing counterfeits. This includes investing in geospatial planning – understanding which populations each facility can actually reach, not just which ones fall within administrative boundaries (tools like Crosscut exist for exactly this).
- Integrated Health Information Systems: Implementing partners and ministries must prioritize patient registries that track individuals across the two-year cycle, send automated reminders, integrate with existing databases, and provide real-time data for planning. Governments and donors should mandate that HIV prevention investments include health information system interoperability – or parallel systems.
- Community Health System Capacity: Governments and donors must invest in training and sustainable compensation for community health workers and peer navigators, community-led monitoring of access barriers and stigma, and culturally competent service delivery designed WITH key populations, not FOR them.
- Sustainable Financing Transitions: Ministries of Finance and Health must begin transition planning now – integrating lenacapavir into national health benefits, allocating domestic resources, and establishing multi-year budgeting frameworks. Donors should require co-financing commitments as conditions of support while providing technical assistance.
- Accountability Mechanisms: We need quarterly progress reporting; an independent scorecard tracking regulatory approvals, supply chain readiness, system functionality, and actual coverage by population and geography; and community-led accountability where key populations monitor whether services are actually accessible, affordable, and stigma-free.
Systems mindset to leverage this moment
Here's what's different about a systems approach: every investment compounds. Money spent strengthening regulatory capacity helps with lenacapavir and every medicine after. Digital patient registries built for HIV prevention can track immunizations, chronic disease, and maternal health. Supply chain improvements benefit every health program. The geospatial planning infrastructure for lenacapavir distribution – tools like Crosscut that map service areas – gets reused for vaccine campaigns, nutrition programs, malaria control.
This is the opposite of vertical, disease-specific programming. We can't afford parallel systems for each innovation. We need foundational infrastructure that makes all innovations accessible.
The lenacapavir agreements demonstrate what's possible when pharmaceutical innovation meets financing creativity meets public health pragmatism. But let's not confuse possibility with inevitability.
The next chapter – where lenacapavir actually reaches the adolescent girl in rural Malawi, the young man in urban Lagos, the sex worker in Mumbai – depends entirely on whether we invest in the unglamorous, essential work of building and strengthening health systems.
The opportunity at hand
And here's the opportunity: if we do this right, if we make parallel investments in systems alongside this breakthrough drug, we don't just deliver lenacapavir. We build infrastructure that delivers better health outcomes across the board, creates more resilient health systems, and makes the next innovation faster and easier to deploy.
Let’s celebrate the breakthrough. Let’s celebrate the investments and pricing agreements. AND Let’s invest in the systems. Let’s fund the infrastructure. Hold partners accountable for systems investments with the same vigor we celebrate drug pricing agreements. Because access without systems is just a well-intentioned press release. But systems strengthened through innovations like lenacapavir create the foundation for health equity that lasts beyond any single drug or program.
The miracle drug is here. The financing is coming together. Now we need the systems to deliver the miracle – and we need them in months, not years. The world is watching. Let's not waste this moment.
Related Posts
GIS Terminology Explained: A Practical Guide for Non-Technical Teams
January 2026 updates: Updated visualizations for cleaner catchment maps





.JPG)
